Which came first, the feather or the bird? The long-cherished view of how and why feathers evolved has been challenged and overturned. It is now believed that feathers evolved in small carnivorous, bipedal dinosaurs - nonavian theropods - that lived on the ground well before the origin of birds and the acquisition of flight.
 |
| This feather from a New England Wild Turkey (from Chestnut Hill, MA) bears a pennaceous distal portion, a plumulaceous proximal portion and intermediary afterfeathers. |
THE GREAT MYSTERY
Feathers are the most complex integumentary structures that have been observed on any vertebrate in earth’s history. Their incredible diversity ranges from the simplest forms to some of the most extravagant pieces of artwork present in nature, and their evolutionary history spans back millions of years to the age when dinosaurs roamed the earth during the late Triassic period of the Mesozoic era. In addition to their striking appearance and ubiquitous nature, there is a great deal of mystery surrounding the origin of feathers.
 |
| From Wikimedia Commons |
The most obvious assumption would be that feathers evolved for flight; however, when we take a closer look at the fossil record we can see that this is not the case. Contrary to popular belief, these incredible structures did not first evolve for flight, but instead evolved in a series of developmental stages that were fueled by a number of evolutionary novelties, the last of which was powered flight.
Feathers evolved in the theropod dinosaurs within the saurischian group, although it is important to note that other dinosaurs outside the theropods have been found with structures bearing resemblance to protofeathers. These taxa include ornithischians such as the heterodontosaurid Tianyulong and the ceratopsian Psittacosaurus.
Although it may seem that the structures between these clades are related to one another, without any evidence in the fossil record of taxa ancestral to both of these groups with primitive feathers, the most reasonable assumption is that these look-alike structures are just an example of convergent evolution.
FALSE LEADS
Although minor variations in the appearance of modern feathers can easily be explained in evolutionary theory, it is much more difficult to explain the appearance of entirely new structures in the fossil record. Due to this difficulty, there have been many hypotheses of the origin of feathers that have been proven unlikely. One of the more popular hypotheses states that feathers evolved from the elongation and division of scales. In this theory, after scales first elongated, they produced fringed edges and then finally branched developing hooked barbules.
 |
| Impression of scales of Tyrannosaurus Rex in sandstone |
However, this theory was proven incorrect by the lack of similarity between the developmental stages of the two structures. As seen below, feathers first develop a cylindrical sheath that later uncurls - meaning the front and the back of the planar sides of the feather stem from the inside and outside of the initial tubular shaft. The planar sides of the scale on the other hand stem from the top and the bottom of the primary epidermal outgrowth that forms the scale. Due to the contrasting developmental nature of these two structures, it is unlikely that feathers could have evolved from scales.
THE FEATHER EXAMINED
So if not scales, what exactly are feathers? When looking closely at feathers, it can be observed that like other integumentary appendages, such as hair, nails and scales, feathers grow from controlled proliferation of cells in the epidermis, which create keratin proteins. In modern feathers, the rachis is the main, tubular shaft of the feather from which individual branches or barbs emanate. These barbs are also branched with tiny-paired filaments known as barbules, which fuse to the shaft of the barb or ramus.
There is also a large amount of diversity in the world of feathers, but these variations can be organized into two blanket groups - plumulaceous and pennaceous feathers. The plumulaceous feather, also known as the downy feather, consists of a simple rachis that hosts a fluffy jumble of barbs, each of which has long spindly barbules attached to it. These feathers are lightweight and provide great insulation. Pennaceous feathers on the other hand are the stiffer aerodynamic feathers that can be observed covering the bodies of birds - the iconic feather of a quill pen. The barbs of these feathers are tightly packed together in a planar fashion.
THE EVOLUTION AND DEVELOPMENT OF THE FEATHER
There are thousands of morphological presentations of feathers represented in the earth’s fauna, varying in size, shape and color; however, each one of these diverse structures that coats the living theropods developed from the same simple design. The feather evolved through a series of incremental morphological changes, which occurred over millions of years.
Stage 1 - a hollow cylinder or protofeather
The first of these stages began from the elongation of the placode (a thickening in the endothelium which gives rise to various integumentary structures), which created a long and rigid hollow tube extending outwards from the skin, the first feather.
This monofilamentous stage of feather evolution is represented by the tyrannosaurid Dilong paradoxus, which was found in the “Feathered Dinosaur Beds” of Liaoning, China. The fossilized tyrannosaur was found to have filamentous integumentary structures as seen below.
 |
| Protofeathers of the tyrannosaurid Dilong paradoxus Traces of filamentous integumentary structures on the caudal vertebrae with line-drawings below. From Xu et al, 2004. |
Stage 2 - unbranched barbs attached to a calamus
The second stage of feather evolution began with the differentiation of the follicle collar. In this stage, the follicle collar split into an inner layer of longitudinal barbs, and an outer layer that consisted of a protective sheath, making up the calamus. From the calamus there extended a tuft of branched barbs, creating the first structure visually reminiscent of a plumulaceous feather.
This morphotype is known from the basal dromaeosaur Sinornithosaurus millenii. The feathers from this taxon are diffusing arrays of filaments lacking a prominent central rachis.
 |
| The filamentous feather of Sinornithosaurus millenii (Chinese bird-lizard) from the dorsal surface of the snout that converge at a single base with accompanying line-drawing. From Xu et al, 2001. |
 |
| Sinornithosaurus millenii from the Early Cretaceous of China From picsearch.com |
Stage 3 - a planar feather with central rachis or with barbules attached to calamus
The next evolutionary stage of development has two potential steps. It is unclear through the fossil record which one of these two evolutionary steps occurred first; however, both of these newly developed feathers would eventually lead to the formation of double-branched feathers, which consist of a rachis as well as both barbs and barbules. These feathers were planar as well as open pennaceous, meaning that the barbules did not yet interlock to create a closed vane.
The first possible step (uppermost feather above) of this third stage is the origination of barbules extending from each individual barb in the tuft. A taxon bearing this variation of the feather is the basal therizonosaurid theropod, Beipiaosaurus. The feathers of this taxon are broad and filamentous feathers that have been elongated.
 |
| In the therizinosaurid Beipiaosaurus each individual feather is represented by a single broad filament. The images above show their attachments to the theropod's tail. From Xu et al, 2008. |
 |
| The sickle-clawed, primitive therizinosaurid Beipiaosaurus from the Early Cretaceous of China From Wikimedia Commons |
The second step of stage three in feather evolution is the helical growth of barb ridges, which lead to a planar feather with unbranched barbs fused to a newly formed central rachis.
Stage 4 - a closed symmetric pennaceous vane
At stage four, the differentiation of barbules began to take place. This led to the formation of hooked barbules, which allowed for an interlocking mechanism between adjacent barbs, thus creating the closed feather vane, and the fourth step in the pathway to flight feathers.
This stage can be observed in a close relative of the Archaeopteryx, the theropod Caudipteryx (meaning "tail-feather"), of which feather impressions have been identified as having an obvious rachis as well as a herringbone pattern within the barbs that is accepted as a close-vaned feather. The closed-vane surface of this feather, impermeable to air, later assisted in enhancing the effectiveness of each wing stroke when birds finally developed flight, and allowed for many of the specializations we see in stage five of feather evolution, such as the formation of the asymmetrical feather.
 |
| The speedy Caudipteryx possessed a closed-vaned feather but could not fly. |
 |
| Peacock-sized Early Cretaceous Caudipteryx from China From Wikimedia Commons |
Stage 5 - a closed asymmetrical vane
In the last stage of feather evolution, the asymmetrical flight feather, resembling the feathers of many modern birds alive today, began to develop. This occurs through the addition of barbs to just one side of the rachis.
Only the most advanced feather shapes have the ability to facilitate flight. The narrow leading edge (outer vane) of the asymmetrical feather is stiff and thin, while the trailing edge (inner vane) behind is flexible and long. This configuration allows the bird to use the tilt of its wings to create lift by adjusting the airflow around them. For this reason, the last step in feather evolution, the asymmetrical feather was crucial to the appearance of flight in the theropod dinosaurs.
MOLECULAR SUPPORT FOR FEATHER EVOLUTION
When looking at the development of the feather, it is also important to consider what is happening on the molecular level, and how that results in such complex structures. In the case of the feather, there are two important genes that assist in pattern formation - sonic hedgehog (Shh) and bone morphogenetic protein 2 (Bmp2). These genes have an essential role in development and are used repeatedly for the creation of the feather starting with cell proliferation, which is induced by the Shh protein. Bmp2 then helps regulate the extent of proliferation and assists in cell differentiation.
In the newly created placode, the Shh and Bmp2 proteins are expressed in a polarized anterior-posterior fashion, and then subsequently expressed at the tip of the cylindrical feather-germ to facilitate elongation. Next, the genes are expressed in the epithelium separating the barb ridges that are beginning to form, and they begin to establish a growth pattern for each ridge. In pennaceous feathers, the signaling of Shh and Bmp2 proteins then lay down the pattern needed for the helical growth of barb ridges, and the formation of the rachis. In plumulaceous feathers the proteins create a pattern for the growth of barbs much simpler than that in pennaceous feathers. This signaling pattern can be observed in the following figure.
 |
| The different expression of the two proteins, Bmp2 and Shh, in disks of skin cells called placodes in embryonic reptiles including birds. From bio.miami.edu/dana/107/107F11_16.html |
CREATING AN EVOLUTIONARY MODEL
So, if powered flight did not come into play until the very end of feather evolution, what fueled the initial changes that brought it to that point? There is still much controversy on this topic, as it is highly difficult to test; however, there are many hypotheses of different evolutionary novelties that seem to make sense in pushing the development of feathers to the extreme.
One of these initial functions was suggested to be thermal insulation, as the long tangled barbules of the plumulaceous feather provide great lightweight coats, and provide their insulation much more efficiently than reptilian scales. This insulation also helps in incubating eggs; therefore, brooding is also considered a possible function of these non-avian feathers.
Water repellency would have been another use of feathers with a closed vane, as the interlocking nature of the barbicels creates a barrier against water. Sexual signaling is also an important function of feathers; feathers can be used as species recognition as well as to send visual signals for sexual or social behavior. Many modern birds use their vibrantly colored displays to inform potential mates they are healthy and physically fit, making them better candidates for reproduction; for this reason, sexual selection tends to favor such elaborate traits.
OTHER ADAPTATIONS FOR POWERED FLIGHT
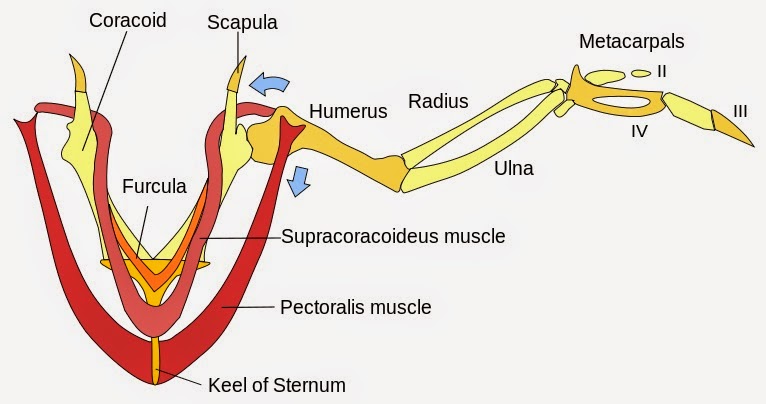
When discussing the evolutionary path to powered flight, there are many anatomical requirements and system modifications that must be considered in addition to the asymmetrical flight feather. One of these important adaptations is a well-developed pectoral girdle, which provides the force necessary to push the air and oppose gravity, thus propelling the animal forward.
This highly derived pectoral structure was not observed in Late Jurassic Archaeopteyx ("ancient feather or wing"), considered to be transitional between feathered dinosaurs and modern birds. The keeled sternum of the modern bird uses its large surface area for a more effective attachment of the pectoralis muscle that powers the down stroke of the wing. The aerodynamic nature of the keeled sternum also allows avian creatures to glide through the air with less resistance, allowing for better flight stamina.
 |
| Artist's impression of Archaeopteryx from the Jurassic of Germany From The Westside Story |
The development of pneumatic bones in theropod dinosaurs was also extremely important for the facilitation of flight. Pneumatic bones contain air sacs that are hooked up to the bird’s respiratory system. These sacs assist in respiration, as well as create a lightweight skeletal structure ideal for flight.
Feathers were not enough to lift the dinosaurs off the ground; they needed a combination of many other anatomical specializations that favored flight to allow them to facilitate lift off. The lack of some of these adaptations for flight in feathered dinosaurs further emphasizes the idea that feathers could not have initially evolved for powered flight.
THE MYSTERY SOLVED?
The evolution of the feather is an intriguing subject on the list of structures of which the ancestral condition is not entirely obvious upon first glance; however, through the close study of the fossil record, various relationships between modern birds and non-avian theropods can be made. By observing such taxa within the dinosaurian group theropoda, one can arrive at the conclusion that feathers are not an adaptation for powered flight, but rather powered flight is the last of a number of evolutionary novelties that pushed the development of the feather to its highly specialized form.
 |
| From Evolution of Dinosaurs into Birds.com |
Well done, Julia!
WORKS CITED
Brush, A.H., R.O. Prum. 2003. Which Came First, the Feather or the Bird? Scientific American: 86-93
Guo, Y, X. Xu. 2009. The Origin and Early Evolution of Feathers: Insights from Recent Paleontological and Neontological Data. Vertebrata Palasiatica: 312-320
Jia, C, M.A. Norell, X. Kuang, X. Wang, Q. Zhao, X Xu. 2004. Basal Tyrannosaurids from China and Evidence for Protofeathers in Tyrannosaurids. Nature 431: 680-683
Poore, S.O., A. Ashcroft. 1997. The Contractile Properties of the M.Supracoracoideus in the Pigeon and Starling: A Case for Long-axis Rotation of the Humerus. The Journal of Experimental Biology 200: 2987-3002
Prum, RO., 2002. The Evolutionary Origin and Diversification of Feathers. Quarterly Review of Biology 77: 261-295
Prum, R.O., X. Xu, Z. Zhou. 2001. Branched Integumental Structures in Sinornithosaurus and the Origin of Feathers. Nature 410: 200-203
Ruben, J. 1991. Reptilian Physiology and the Flight Capacity of Archaeopteryx. Evolution 45: 1-17
Stettenheim, P.R. 2000. The Integumentary Morphology of Modern Birds-An Overview. American Zoology 40: 461-477
Wake. D.B. H. You. X. Zheng, X. Xu. 2009. A New Feather Type in a Nonavian Theropod and the Early Evolution of Feathers. Proceedings of the National Academy of Sciences of the United States of America 106.3: 832-834
Witmer, L. 1990. The Craniofacial Air Sac System of Mesozoic Birds (Aves). Zoological Journal of the Linnean Society 100: 327-343
Zimmer, C. 2011. Evolution of Feathers. National Geographic Magazine